In a single molecule layer, water can behave neither as solid nor as liquid
We may think we know how water behaves, but we’ve overlooked what happens when it gets into spaces so small that it’s only a single molecule thick. The results turn out to be very different from water in bulk.
water’s three known phases of matter – solid, liquid and gas – can lead to icebergs when they meet. Like its metaphorical counterpart, however, there’s a lot more to it where we can’t see it, including countless phases of matter that only exist under extreme conditions. Be more of this often discovered while learning how to manipulate and measure matter in new ways.
The latest examples occur when water is trapped under conditions where it forms a sheet with a thickness of one molecule, at which point several phases can occur in sequence. Unlike some other phases of matter, not all of them require enormous amounts of energy or pressure to make. Instead, the challenge lay in observing their behavior and understanding the structures, which has now been addressed in a paper published in Nature.
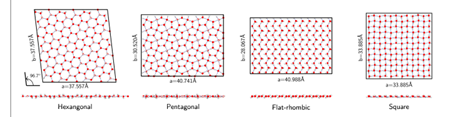
Using computational modeling of water within a small graphene channel, Dr Venkat Kapil from the University of Cambridge and colleagues found at low pressure, the water forms a phase similar to bulk ice — except it has a melting point about 100°C lower than the three-dimensional version — with molecules arranged in a hexagonal fashion.
As the pressure or temperature increases, the molecules rearrange themselves into first pentagonal and then rhombic shapes. At pressures the authors call “average” (about 8,000 atmospheres), the water enters what the authors call the “hexatic” phase. Here, water behaves somewhere between solid and liquid, with solid but rotating molecules, until the temperature rises above about 70°C (158°F).

Schematic representation of the way in which the water molecules arrange themselves under different pressures in a water monolayer. Image credit: Kapil et al/Nature
As the pressure increases further, the water becomes superionic. It looks more like ice than water, but is highly electrically conductive. However, the current is not carried by protons, not by electrons. Bulk water can also have a superionic phase, but you need much higher pressures to achieve this.
Other phases of matter, such as those occurring at very low temperatures or under extreme pressure, are sometimes of more curiosity than practical application, but that is not necessarily the case here. Small holes occur in all kinds of porous materials and thick layers of one molecule can form between membranes, whether we need them or not.
The authors argue that our own bodies contain some of these phases when the spaces inside are too small for bulk water, and their response could affect the effectiveness of medical treatments. Similarly, batteries and water desalination projects have included water in these stages for a long time, we just don’t know how it affects production.
“For all these areas, understanding the behavior of water is the fundamental question,” Kapil said in a statement pronunciation. “Our approach enables the study of a single layer of water in a graphene-like channel with unprecedented predictive accuracy.”
The hexatic phase behavior reported by the authors is largely consistent with previous predictions, but the superionic takes us further into uncharted territory.
“The existence of the superionic phase under easily accessible conditions is peculiar, as this phase is generally found in extreme conditions such as the nucleus of Uranus and Neptune. One way to visualize this phase is that the oxygen atoms form a solid grid, and protons flow through the grid like a liquid, like children running through a maze,” Kapil said.
The authors hope to exploit the exceptional conductivity of the superionic phase to improve battery design.